Soil Acidification: An Emerging Problem in MT
by Clain Jones, Richard Engel, Stephanie Ewing, Perry Miller, Kathrin Olson-Rutz and
Scott Powell
Land Resources and Environmental Sciences, MSU, Bozeman
INTRODUCTION
Farmers in several Montana counties are experiencing nearly complete yield loss in portions of their fields due to soil acidity (low pH). Traditionally, soil acidity was not an issue in much of Montana as most soils in agricultural areas had naturally high pH. However, this is changing.
EXTENT
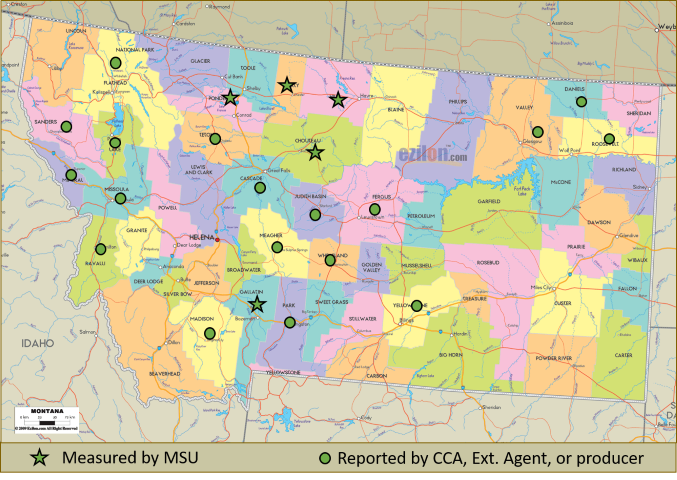
Figure 1. Counties in Montana with a least one field with top 0-6” soil pH < 5.5, Dec 2018. Symbol is not the location of that field.
Montana State University soil scientists, Extension Agents, crop advisers, and producers have now identified fields in 23 Montana counties with locations where the top 0 to 6 inches of soil have pH below 5.5 (Figure 1), some as low as 3.8. Soil acidification has been reported elsewhere in the northern Plains region and Pacific Northwest.
CAUSES
There are many natural and agronomic reasons for low soil pH (see text boxes). The primary agronomic cause is use of ammonium based nitrogen (N) fertilizers (e.g., 46-0-0, 32-0-0, 28-0-0) at rates that exceed crop N uptake. When the ammonium gets converted to nitrate, hydrogen ions (H+)
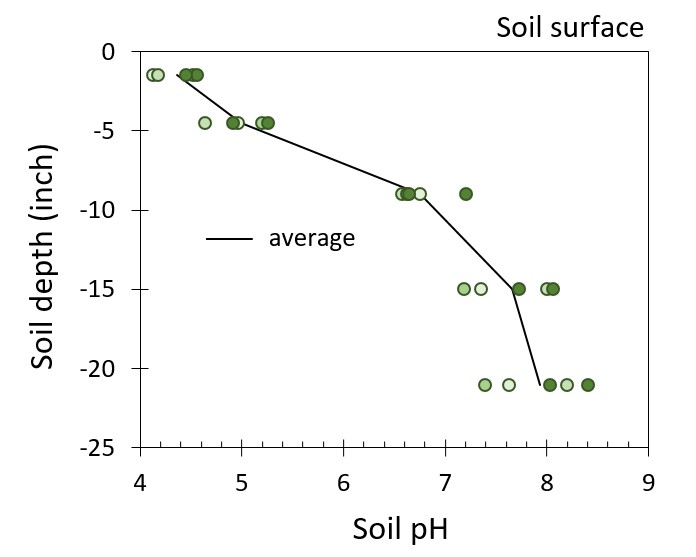
Figure 2. Soil pH down the soil depth profile of 5 farm fields located across north-central MT (R. Engel unpub. data).
are released, lowering soil pH in the top soil layer (Figure 2). If nitrate is taken up, plants release basic anions (OH- and HCO3-) to maintain their charge balance. Conversely, if nitrate leaches, these basic ions are not released and are then not available to balance the acidity coming from ammonium to nitrate conversion. Since 1985, there has been an almost 3-fold increase in N fertilizer use in Montana, largely to achieve high wheat grain protein for increased profit. This increase in N applied has accelerated soil acidification. In addition, no-till direct seeding operations have become the norm for Montana dryland farmers. The absence of tillage contributes to pH stratification and acidification of the soil layer receiving N fertilizer, while underlying soil material remains relatively unaffected.
| Soils with low buffering capacity, low soil organic matter, coarse texture, granitic parent material |
|---|
| Historical forest vegetation |
| High precipitation, leading to more leaching, higher yields and higher N fertilization |
|
Ammonium-based N fertilization above plant uptake ammonium or urea fertilizer + air + H2O → nitrate (NO3-) + acid (H+) |
|---|
|
Nitrate leaching = less nitrate uptake and less root release of basic anions (OH- and HCO3-)
|
|
Crop residue removal, removes base cations (calcium, magnesium, potassium)
|
|
No-till concentrates acidity in soil layer where N fertilizer applied
|
|
Legumes acidify their rooting zone during N fixation, yet apparently much less than
fertilization of wheat
|
We have conducted a few long-term cropping systems studies with records of N inputs. In one study, after 14-years, soil pH was 6.2 under a continuous wheat system that received recommended N rates (~3 lb available N/bu, 2050 lb N/acre cumulative) in contrast to soil pH 7.3 in the organic wheat system that used legume green manure instead of N fertilizer. This implies that even a neutral to alkaline soil can become acidified over time by N fertilization.
Acidification rate varies with soil texture. In the 14-year trial on a silt loam, each 100 lb N/acre produced a 0.044 drop in soil pH in the top 4 inches (Engel et al. unpub. data). The potential acidification is greater on coarser soil. A 6-year study on a sandy clay loam found urea fertilization at suggested rates of 3 lb available N/bu produced an alarming 0.15 soil pH drop in the top 3 inches for each 100 lb N/acre applied (Jones unpub. data).
IMPACT
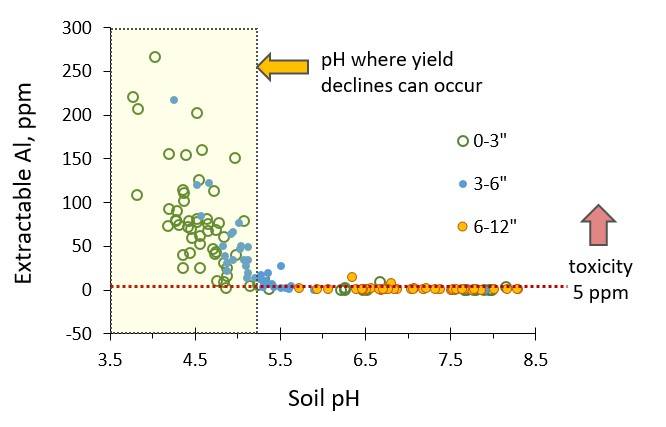
Figure 3. Available aluminum and soil pH at 3 soil depths at 5 Montana farms, illustrating why problems begin near pH 5 (R. Engel unpub. data).
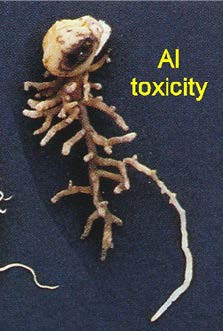
Acidic soils reduce crop production several ways. The major impact we have found is aluminum (Al) toxicity (Figure 3). At low pH, some nutrients (for example, potassium) are less available to plants. Acidic soils also change the efficacy and persistence of herbicides (Raeder et al. 2015). Unexpected herbicide carryover may be an indicator of drops in soil pH, because some herbicides break down more slowly at low pH. Nitrogen deficient legumes may be another indication of acidified soil, since N-fixing rhizobia do not thrive at pH less than 6. Finally, fungal diseases become more prevalent at lower soil pH, and hydrogen ions as well as manganese (Mn) can reach toxic levels (Kidd and Proctor 2001). Aluminum toxicity leads to club roots, small leaves, with yellow, purple or brown lesions and leaf withering in the center (Figure 4). See https://landresources.montana.edu/soilfertility/nutrientdeficient/ for more photographs.
The impact is rarely uniform across a field (Figure 5). Soil pH can vary greatly across short distances. Low lying or toe-slope areas often have the lowest surface-layer soil pH, and pH may be 2 units higher on or near a ridge less than 100 yards away. Ongoing MSU research is determining appropriate soil testing procedures and prevention, adaptation, and mitigation options, which will be discussed in a future Fertilizer Fact. More information is available at MSU's Soil Acidification Webpage, and in The Soil ScoopsSoil Acidification: Problems, Causes & Testing, and Soil Acidification: Management.




FERTILIZER FACTS
- Traditionally neutral to alkaline soils are becoming acidic in the top 6 inches, primarily due to the use of ammonium based fertilizers.
- Acidic soils lead to crop yield losses because of aluminum toxicity, reduced N fixation in legumes, changes in herbicide efficacy and persistence, and increased fungal disease.
ACKNOWLEDGMENTS
Montana studies were funded by the Montana Agricultural Experiment Station, the Montana Fertilizer Advisory Committee, and USDA’s Western Sustainable Agriculture Research and Education program. We thank collaborating producers for allowing us to conduct research on their farms.
REFERENCES
Kidd, P.S., and J. Proctor. 2001. J. Exp. Bot. 42:791-799. doi:10.1093/jexbot/42.357.79
Raeder, A., et al. 2015. How Soil pH Affects the Activity and Persistence of Herbicides. Washington State University Extension FS189E
Edited by Clain Jones, MSU Extension Soil Fertility Specialist, and Kathrin Olson-Rutz, Research Associate
Posted March 2019