Pea Protein Formation and Management Options
Mike Bestwick1, Perry Miller2, Clain Jones3, and Kathrin Olson-Rutz4
1. Former MSU Research Associate; currently with Jackola Engineering and Architecture, Kalispell, MT, 2. Professor, 3. Professor/Extension Soil Fertility Specialist, and 4. Former Research Associate, Dept. Land Resources and Environmental Sciences, Montana State University, Bozeman.
This 2018 review covers protein formation in pea as well as what is known about nutrient management strategies’ effect on pea protein content. A printable PDF version of this report is also available.
Introduction
The pea protein market is expanding. In 2015, the U.S. pea protein market was valued at $22.8 M, and is projected to exceed $34.0 M by 2020 (Markets and Market, 2015). Market growth is being driven by consumer preference for vegetarian, non-GMO and gluten-free sources of protein which has given pea a marketing advantage over dairy and soy-based protein sources. Further, new processing technologies have made pea protein extraction efficient and affordable.
Examples of major food companies that use pea protein in their products include (Fig 1):
- General Mills - Larabar ALT Protein Bar
- Hampton Creek - Just Mayo
- Now Sports - Pea Protein
- Kirkland - Nature's Domain Dog Food
- Barilla - ProteinPlus Pasta

Figure 1. Food products in which pea protein is a key ingredient.
Montana led the nation in dry pea production in 2016 with nearly 600,000 acres harvested (NASS, 2017). If consistently high protein content can be maintained in Montana-grown pea, the pea protein industry may target and pay more for Montana grown pea, which could increase producer revenues.
Protein content hinges on growing conditions and management. Montana’s major agricultural regions are characterized as semi-arid with highly variable precipitation and temperature patterns (Padbury et al., 2002). Pea is typically seeded in April and terminal drought forces the crop to mature by July, which means drought will affect physiological processes related to pea protein formation. Management options may also affect protein formation, with the use of inoculant and fertilization being the most pertinent to Montana.
As the protein market continues to expand, studies addressing how management affects pea protein in drought-prone regions will become increasingly important in helping Montana producers decide how to manage pea for high protein. For instance, understanding how protein is influenced by different fertilizers, inoculant types, or crop rotations in a wet or dry growing season could help producers assess the financial risk of various management plans under climatic uncertainty. The objective of this document is to a) provide a basic physiological framework for protein formation in pea under ideal, drought, and nutrient-deficient growing conditions, and, b) present information on management effect on pea protein in drought-prone regions similar to Montana.
Protein Formation in Pea
Pea protein content is directly related to seed nitrogen (N) content—high seed N means high protein. At the plant level, pea protein depends on plant N uptake and remobilization of N to seeds. Under ideal growing conditions—meaning water and nutrients do not limit pea growth—soil nitrate (NO3-) is initially taken up by pea roots and stored in shoots and leaves until pods begin to fill. During pod-fill, roughly 60% of N stored in shoots and leaves is remobilized to the seed, while around 50% of N being taken up at that time is sent directly to the seeds (Schiltz et al., 2005; Fig. 2.). Final seed N concentration is lastly determined by seed number—with fewer seeds per plant, final seed N is more concentrated and protein content is higher.
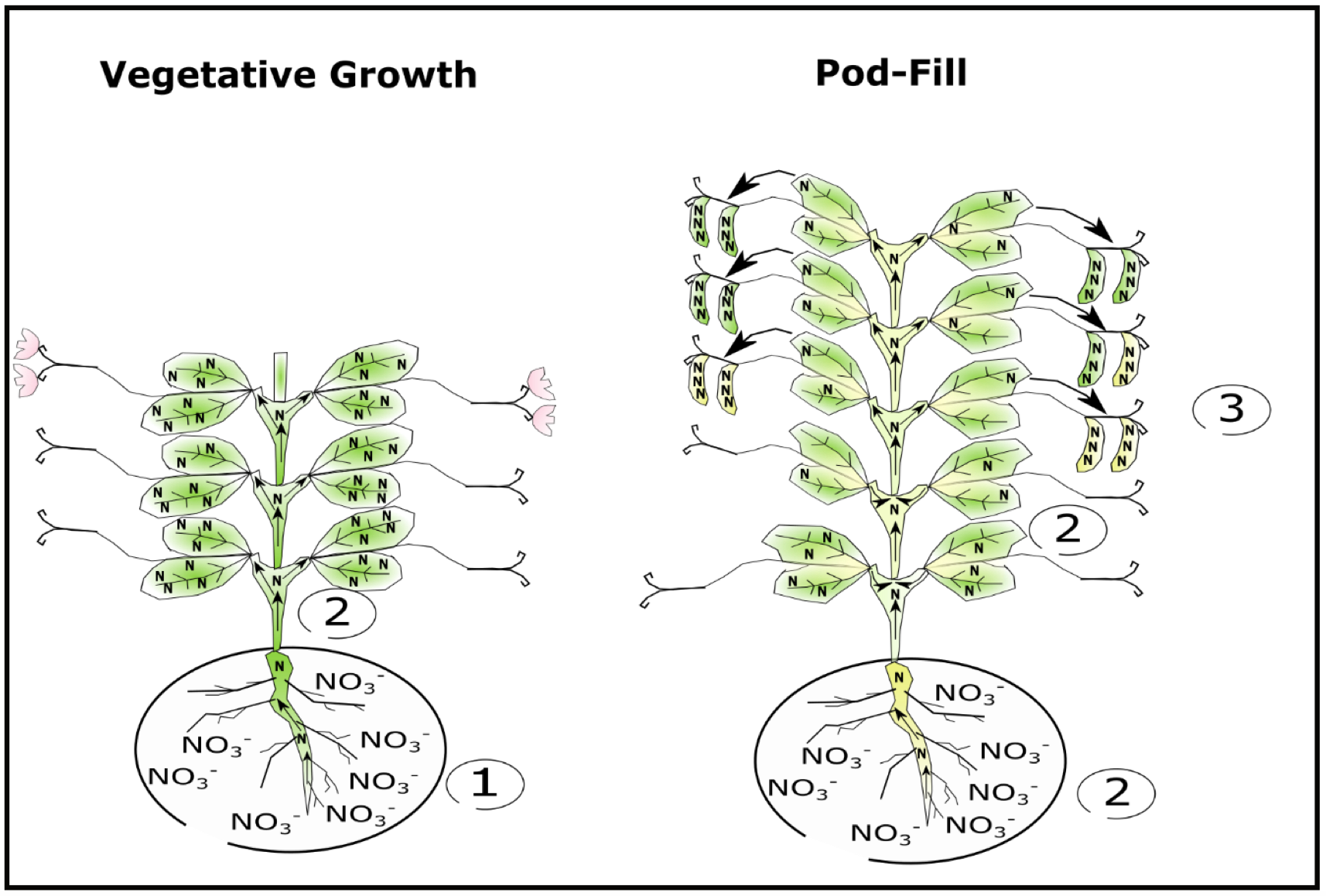
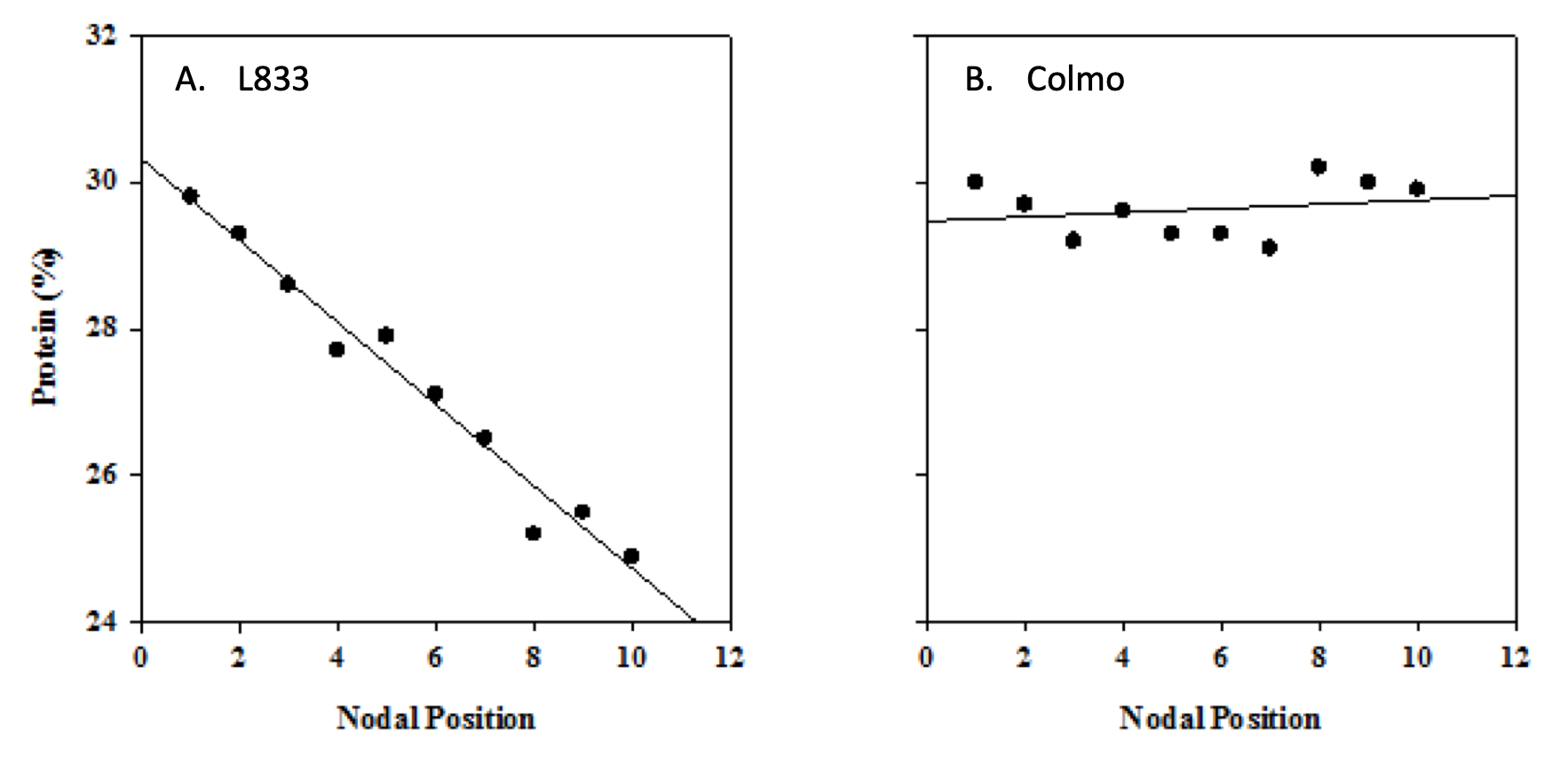
Differences in plant genetics may play a role in dictating final seed protein. For instance, varieties that produce fewer seeds are more apt to produce high seed protein (Lhuiller-Soudele et al., 1999). Similarly varieties that are more efficient at remobilizing shoot and leaf N to seeds during pod fill may produce higher protein compared to less efficient varieties (Larmure and Munier-Jolain, 2004). Because pea seeds successively develop from the lowest to highest nodes, individual seed protein can be affected by nodal position and variety. Atta et al. (2004) observed that individual seed protein decreased from 29.8 protein units in the first developed or lowest node to 24.9 protein units in the tenth or highest node in the French variety L833 (Fig. 3A). Alternatively individual seed protein remained constant across nodes in the variety Colmo (Fig. 3B). Lower individual seed protein at upper nodes in L833 was attributed to less efficient N acquisition and remobilization to seeds when pods were filling in upper nodes compared to when seeds were developing in lower nodes. In Colmo, N acquisition and remobilization remained constant when seeds were developing at both upper and lower nodes due to better maintenance of biological N fixation during pod development.
Despite the potential for variety to affect protein, environmental factors may have a greater effect on the physiological processes that affect protein formation for pea grown in Montana (Bestwick, et al., 2018; Tao et al., 2017). For instance, drought stress during pod development may reduce seed number which in turn could affect final seed N concentration. Earlier drought stress may compromise N fixation at an earlier growth stage (Marino et al., 2007), and limit the N uptake by the pea plant altogether. Water, nutrient stress, especially N, or a combination of the two are common in Montana and are likely to affect protein content.
Drought Stress and N Deficiency on Protein
Water stress
Drought stress results from low or poorly timed precipitation relative to important crop growth stages. In pea, drought stress before flowering reduces vegetative biomass. Reduced vegetative biomass translates to less stored N in stems and leaves, which means less potential for N to be remobilized to seeds during pod- fill (Lhuiller-Soudele et al., 1999). Alternatively, drought stress incurred at any period over the crop cycle can reduce seed number (Guilioni et al., 2003), thus boost protein. Depending on timing and severity of drought, it is possible that drought stress could increase, decrease, or have no effect on pea protein compared to a well-watered plant (Fig. 4). Under dryland conditions in Montana, drought stress in spring pea occurs must commonly at or after flowering, and therefore may have a general effect of increasing seed protein (P. Miller, pers. comm.). However, in 2017, drought stress in the Montana/North Dakota region occurred during early vegetative stages and consequently many producers experienced very low seed protein levels.
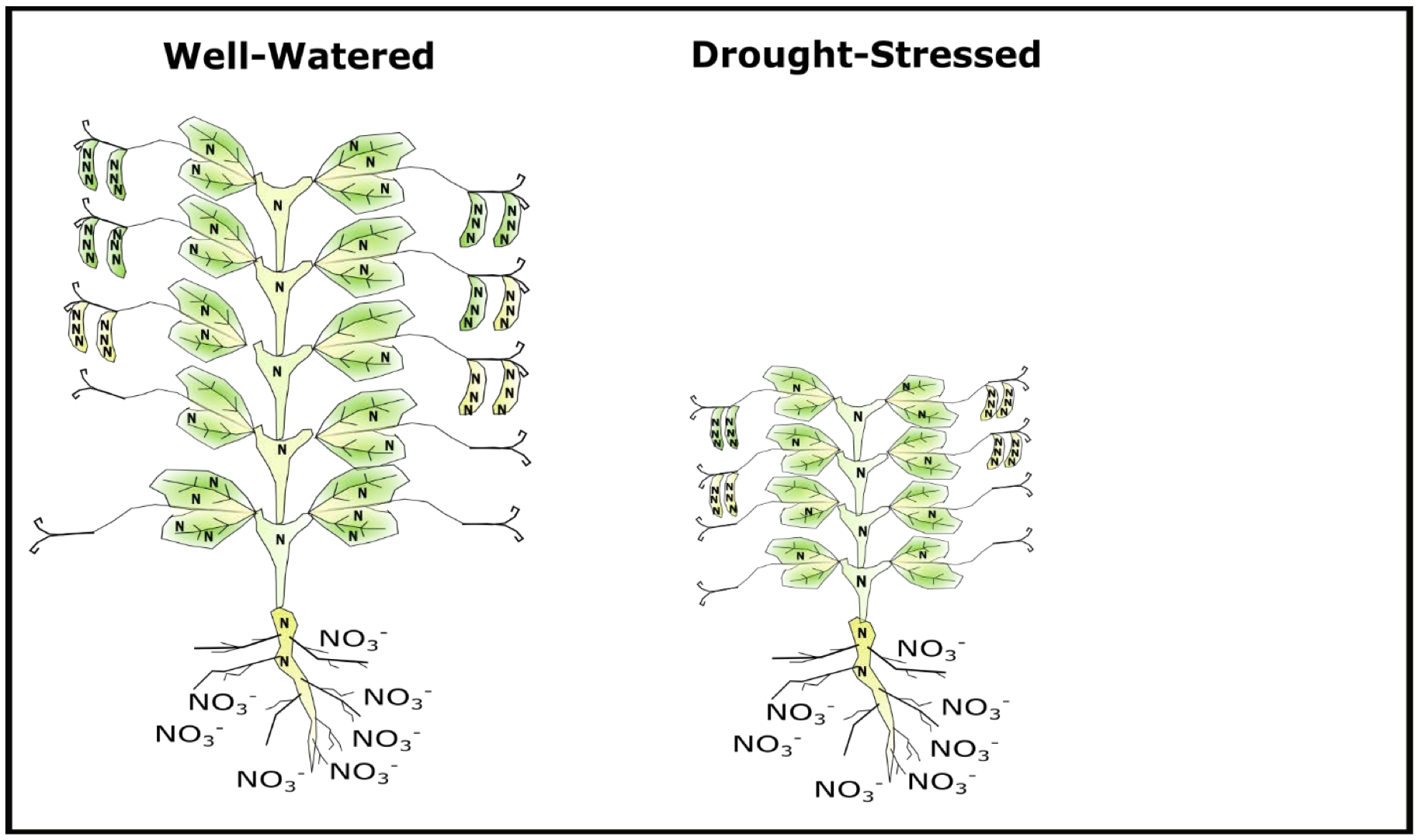
N stress
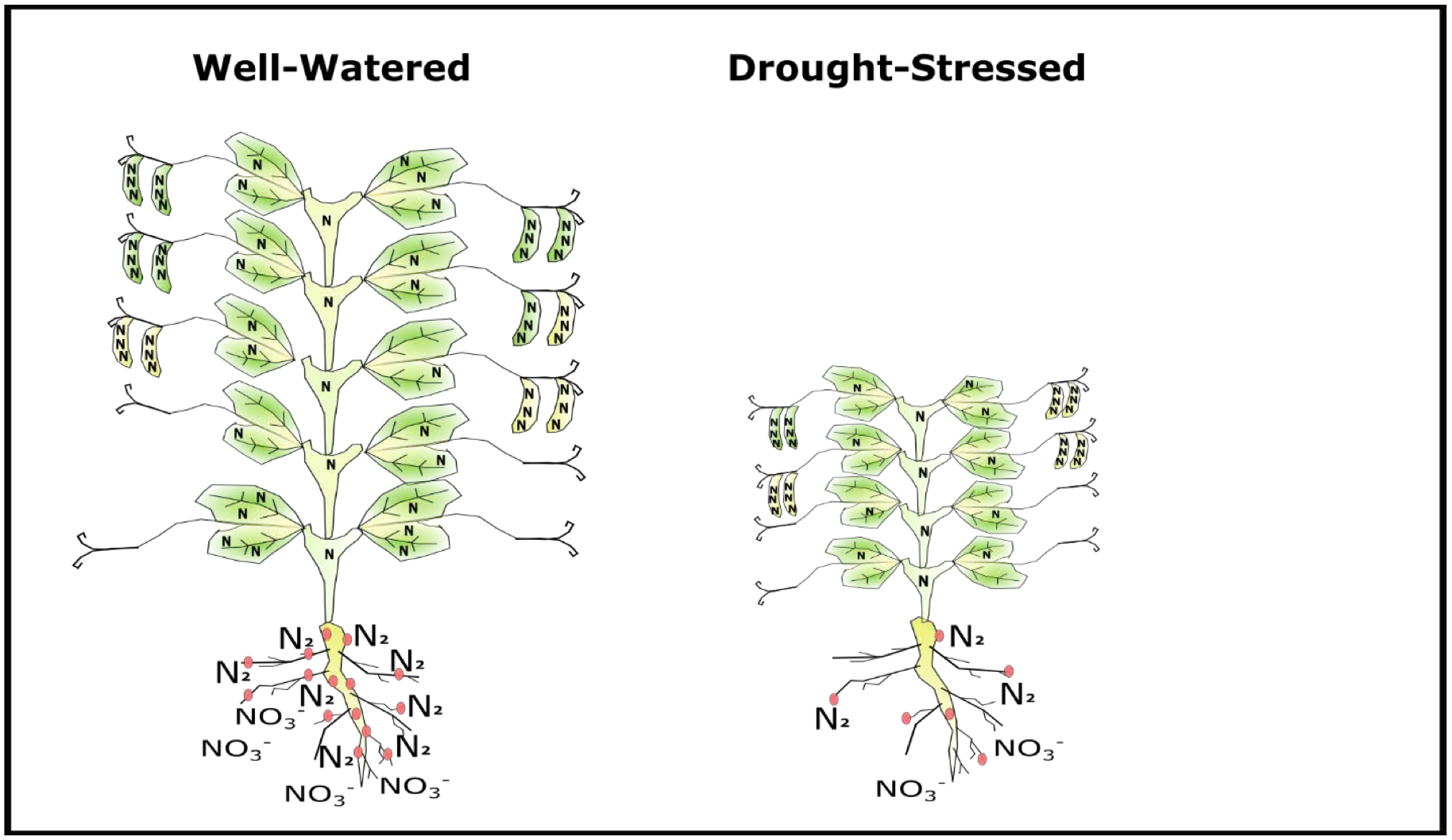
Nitrogen availability is tightly related to water availability. Because no or low rates of fertilizer N are applied to pea, N becomes plant available primarily through decomposition of organic matter and crop residue and N fixation. Under wet and warm growing conditions, soil microbial activity needed to decompose organic N into nitrate for plant uptake is high. Moist (but not saturated) soils also support a healthy environment for rhizobia populations which maintain nodule activity for effective N fixation.
Conversely, decomposition and N fixation rates are reduced when soils are dry or cool. This means less N will be made plant available through decomposition or N fixation in a low rainfall year compared to moist conditions, but final protein depends on the extent to which drought also reduced plant biomass and seed number (Fig. 5).
N Management
Despite the complexity of how drought and N interactions affect pea protein, a number of studies have addressed how protein responds to different N management strategies in semi-arid systems similar to Montana. Specific N management strategies include applying different N rates, use of inoculation, and selecting inoculant type.
Starter N
Early studies conducted in the Canadian prairies suggest protein can be increased at high N-rates (Sosulski et al., 1974; Holl and Vose, 1980) regardless of wet or dry growing season conditions. For instance, Holl and Vose (1980) showed that N rates greater than 143 lb/acre increased protein by approximately 6 protein units compared to an unfertilized control in subhumid northeastern Saskatchewan. This result was attributed to greater seed N accumulation during the pod fill period (Fig. 6A). Likewise, McLean et al. (1974) showed in a greenhouse study that seed protein increased from 20 protein units with 0 lb N/acre to 30 protein units with 293 lb N/acre (Fig. 6B).
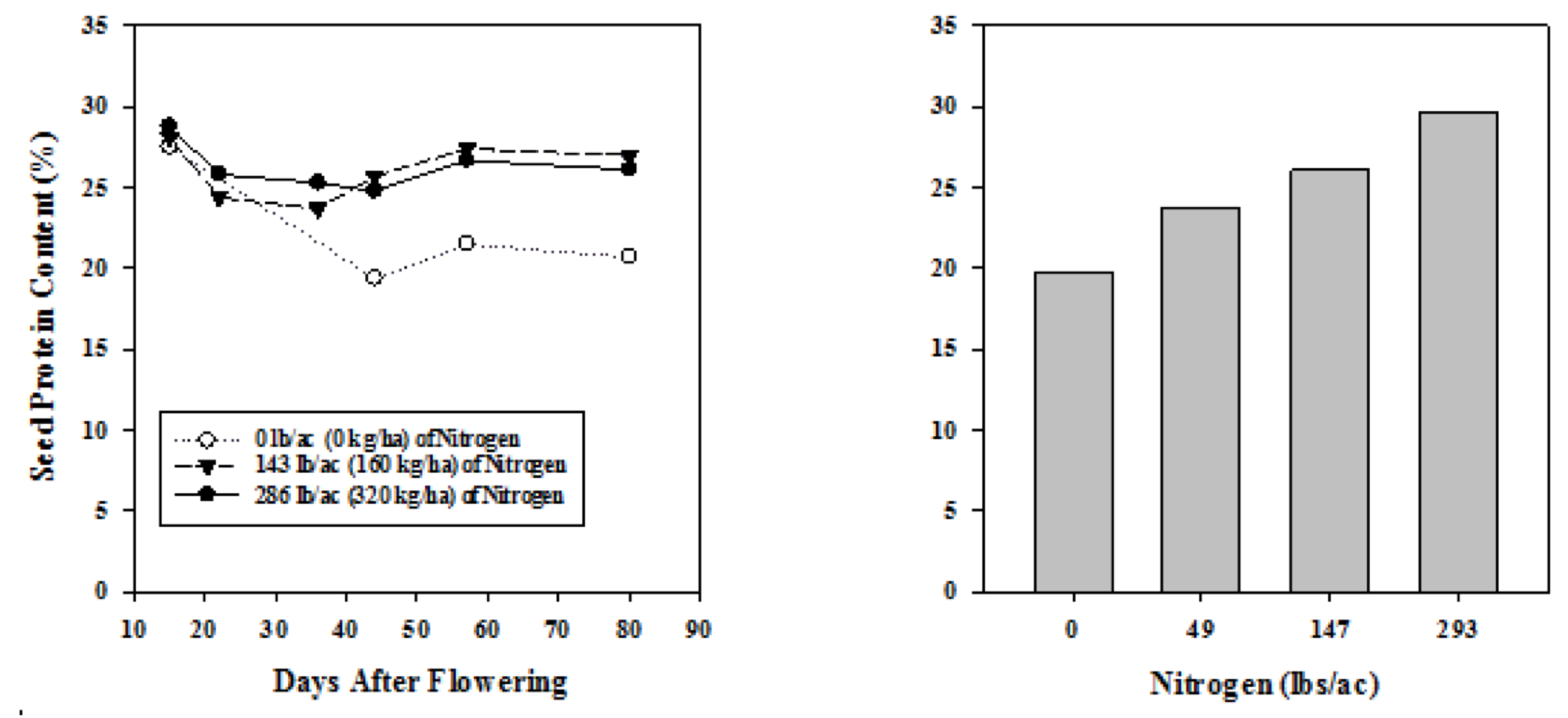
However, such high levels of starter N are not practical. Nitrogen fertilizer is the greatest input cost for crop production in Montana, and it is unlikely that increased pea protein would offset fertilizer costs— particularly at rates greater than 143 lb N/acre, and, since pea is marketed as being a low input crop in terms of nutrient requirements, high N fertilizer may deteriorate pea marketing potential. Also, improvements in seed inoculant has increased N fixation potential (Rennie and Hynes, 1993) which may greatly reduce or even eliminate the need for fertilizer N. A Montana study found that inoculated pea could fix 49 to 112 lb N/acre depending on growing season conditions (McCauley et al., 2012). For these reasons, recent work has focused onhow economical starter N rates and/or inoculant types (i.e., granular vs. seed-coat) affect protein.
Although legumes can fix their own N, there are several reasons to provide low levels of N fertilizer. Nodulation requires healthy plants. If there is little soil nitrate, the nodulation process can become ‘stuck’; unable to grow to feed nodules, and nodules don’t form so they are not providing N for growth.
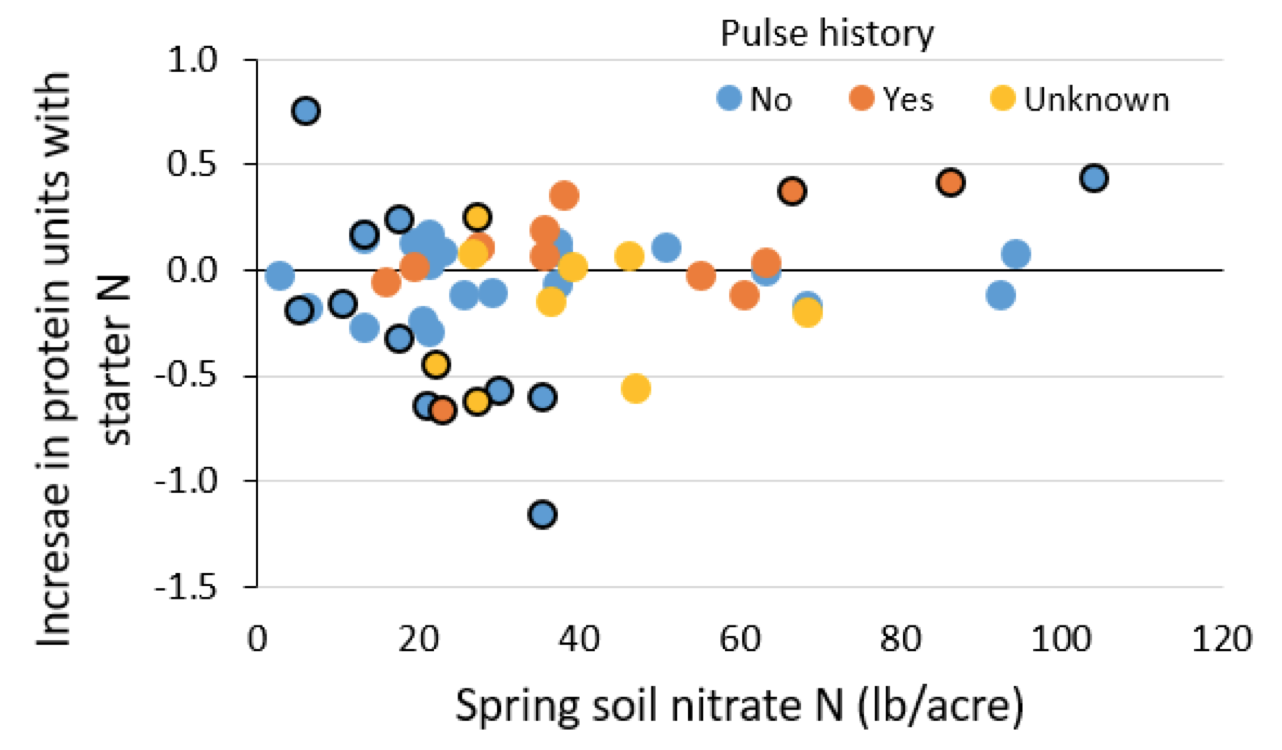
A four-year study conducted across Alberta found starter N did not have a consistent impact on pea protein, regardless of field pulse history or spring soil nitrate-N level (Fig. 7).This is in general agreement with McConnell et al. (2002) and Clayton et al., (2004a) that inoculant is more likely to increase seed protein than starter N.
Inoculation
There are several practices that can improve nodulation and N fixation. It is important to use plant species-specific inoculant applied at the proper rate, although some inoculants are interchangeable among species, such as pea and lentil (but not chickpea). Liquid and peat-powder seed-applied inoculants need to be kept cool and dark before application. Granular inoculants better protect their rhizobia. Contact with fertilizer in mixing or application equipment can kill bacteria. Similarly, some fungicides may not be compatible with inoculants (Kutcher et al., 2002). In traditionally water-limited regions, practices that retain soil moisture, such as no-till, can enhance N fixation.
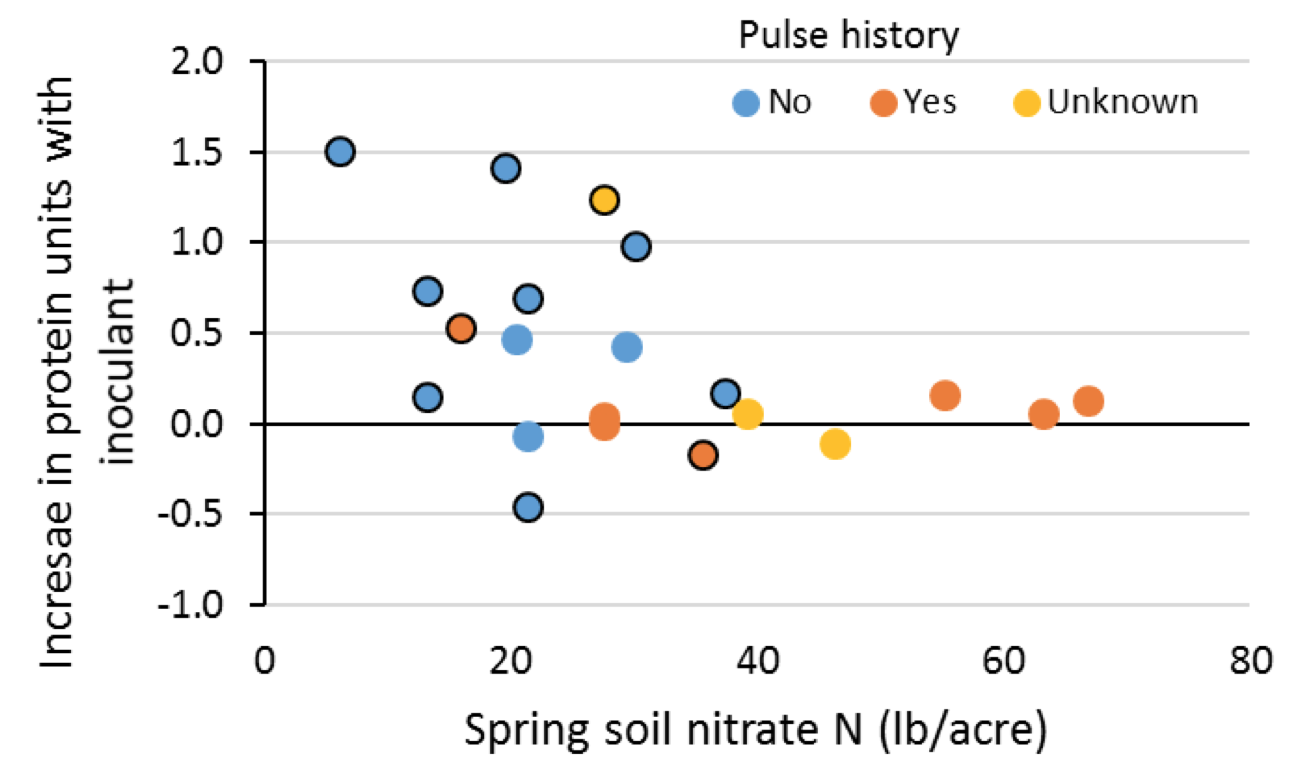
Inoculant is most effective under low spring soil nitrate levels and in fields where legumes have not been grown for at least five years. In an Alberta study of 21 field trials, inoculant was far more likely to boost protein on fields with no pulse crop history than those with crop history and when spring soil nitrate-N levels are less than 18 lb N/acre. Protein is highly unlikely to increase with inoculation under high spring nitrate levels (greater than 36 lb N/acre; Fig. 8).
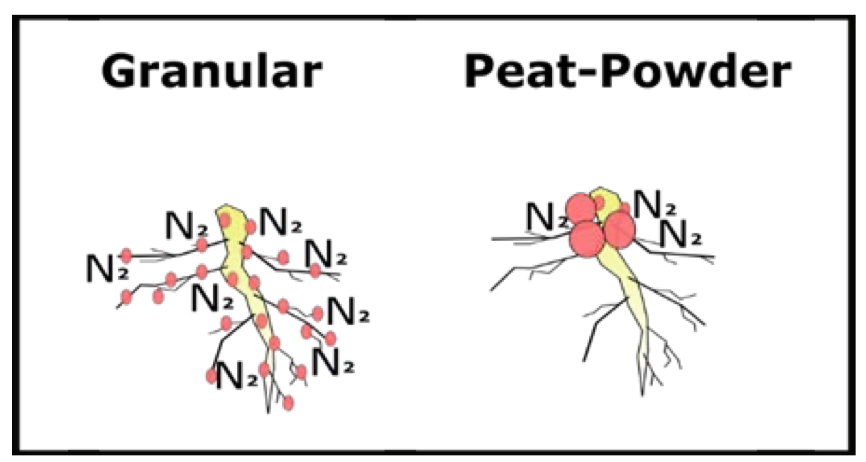
Granular inoculant has been observed to be more effective at fixing N than seed-coat inoculant (Clayton et al., 2004a). N fixation is often superior with granular inoculant compared to seed- coatings due to differences in application methods. Specifically, granular inoculant is applied to the soil at seeding, and peat-powder seed-coat is applied directly on the seed as a thin layer. Soil-applied granular inoculant allows rhizobia populations to be evenly distributed on pea roots whereas rhizobia are clustered near the root crown with seed-coat inoculant. Consequently nodules form on tap and lateral roots from granular inoculant, and larger nodules tend to cluster on the root crown with seed- coat (Fig. 9). The widespread nodule distribution associated with granular inoculant has been attributed to increasing N fixation. Bestwick et al. (2018) found a higher correlation between peaprotein and granular inoculant, than when producers used seed-coat inoculant, especially under drought conditions.
A two-year study conducted at three locations in Alberta showed that seed protein averaged 19.4 and 17.6 protein units using granular and seed-coat inoculant respectively (Clayton et al., 2004b). Starter N was applied at 0, 18, 56, and 72 lb N/acre in this study but had little effect on protein content. It was therefore concluded that increased N fixation from granular inoculant was mainly due to superior nodule distribution on pea roots, and greater N fixation during the pod-fill period may have boosted final seed N and protein.
Greater yield responses were also associated with granular inoculant relative to seed-coat (Clayton et al., 2004b). For information on inoculation, starter N and other nutrient management (P, K, S and micronutrients) on pea yields see our paper published in Crops & Soils Magazine, Volume 51, Issue 4 (https://dl.sciencesocieties.org/publications/cns/tocs/51).
Inoculation Failure: Rescue N
Inoculant generally performs as well or better at maintaining high protein and yield compared to applying fertilizer N. However, inoculant may fail to produce root nodules for effective N fixation for a variety of reasons, including dry or waterlogged conditions, extreme soil temperatures, or rhizobia death during storage and handling. In the case of inoculation failure, applying fertilizer N may be needed to provide pea with adequate N supply for protein and yield formation.
A two-year study conducted at two central Montana sites compared pea protein and yield response to peat granular inoculant and fertilizer N applied 0, 4, 6, and 8 weeks after seeding (McConnell et al., 2002). Protein and yields were consistently lowest for the non-inoculated control, and generally greatest with granular inoculant (Fig. 10, on page 9). In a number of instances, granular inoculant and fertilizer N applied either 0, 4, or 6 weeks after seeding produced similar protein and yield. Hence, the conclusion from this study was that granular inoculant will provide adequate N supply to meet protein and yield potential under normal growing conditions, but if nodulation fails, it may be worth applying fertilizer N within 6 weeks after seeding (up to 7-11 leaf stage) to ensure adequate N supply. Poor nodulation can be detected earlier by digging a few plants and looking for active nodules, which should be visible by the two-leaf stage. Rescue N relies on rainfall to be incorporated into the soil and taken up by the roots.
N Management Summary
Adequate N supply can likely be met by inoculation, and granular inoculant may be more effective than seed-coat. However, low starter-N rates can hedge against ‘stuck’ N fixation. If there is poor nodulation, rescue N may be beneficial if applied by the 7-11 leaf stage.
Neither inoculation nor starter N will consistently increase protein or yield, especially in fields with high spring soil nitrate levels or a rotation history of legumes, but both may be economical insurance against inoculation failure. Spring soil nitrate testing and knowledge of rotation history can influence the decision to apply starter N and/or inoculant.
P, S, and K Management
Although most studies have focused on the effects of inoculation and N management on pea protein, phosphorus (P), sulfur (S) and potassium (K) are known to affect N fixation and could therefore affect protein too. The next sections describe how P and S deficiency limit N fixation and protein.
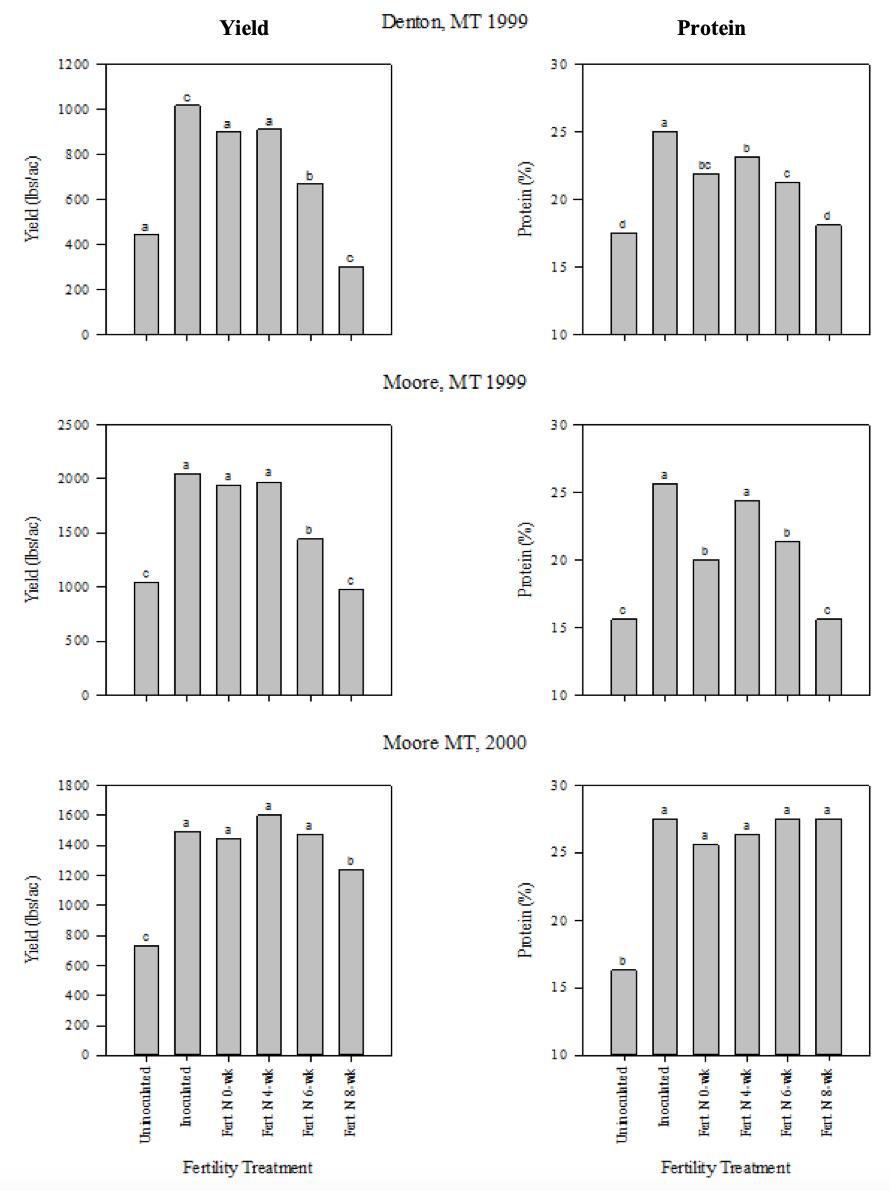
Figure 10. Effect of six fertility treatments on pea yield and protein at two Montana sites over the 1999-2000 growing seasons. Fertility treatments were no inoculation, granular inoculant, and broadcast fertilizer N applied at 0, 4, 6, and 8 weeks after seeding. Different letters above bars indicate statistical differences at the p<0.05 significance level (McConnell et al., 2002).
P Deficiency and N fixation
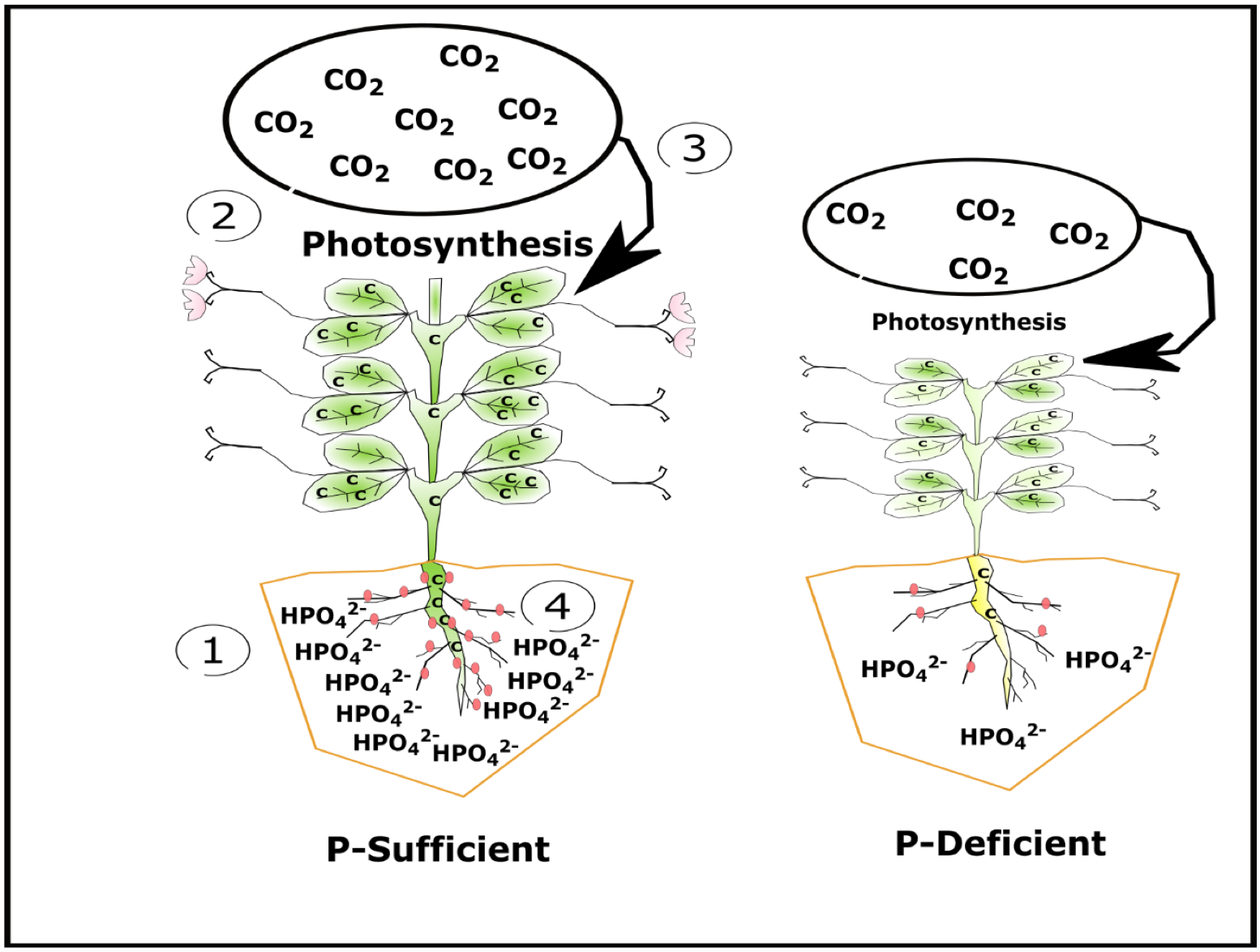
Phosphorus indirectly affects N fixation. Plants deficient in P produce less green-leaf area and biomass which in turn reduces photosynthesis. Reduced photosynthesis limits the amount of atmospheric carbon (CO2-C) assimilated by pea. Because rhizobia living in pea nodules use plant carbon (C) as an energy source to fix N, P deficiency can reduce N fixation (Fig. 11).
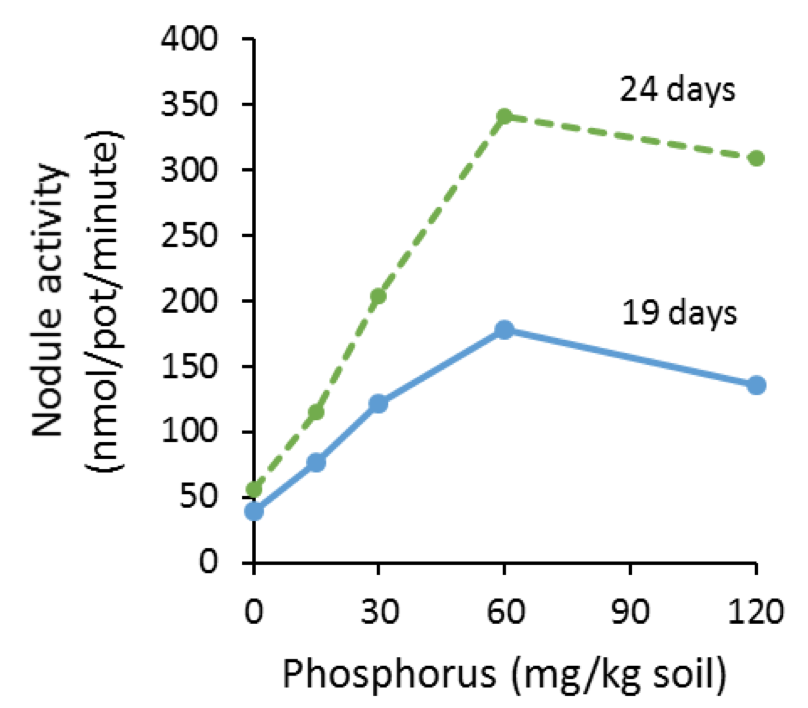
Jakobsen (1985) measured N fixation 19 and 24 days after seedling emergence in a greenhouse experiment in response to P fertility rates (Fig. 12). Pea nodule activity increased with P availability, to a point.Higher N fixation from P was attributed to increased photosynthesis. Since available P can increase N fixation, starter P could affect protein.
Starter P
An early Saskatchewan study found that starter P boosted protein by 1.7 protein units with 52 lb P2O5/acre compared to no applied starter P (Sosulski et al., 1974). A more recent province-wide study in Alberta showed that starter P at rates up to 52 lb P2O5/acre only increased protein on average by 0.2 protein units across 52 sites (McKenzie et al., 2001b). At sites with spring soil P tests below 15 ppm in the top 6 inches, the average increase in protein was 0.4 protein units with starter P.
The Alberta study also showed that starter P could boost yields, likely due to low soil P test levels. Starter P boosted yields at 19 of 52 sites (36%), with an average yield increase of 7% over the non-fertilized controls. Yield increase was much more likely on sites with soil P test levels below15 ppm (52%) and a third of the sites with low P had yield increases greater than 15%. Combined, these studies suggest there is potential for slight increases in protein and yield with starter P when soil test levels are low.
S Deficiency and N fixation
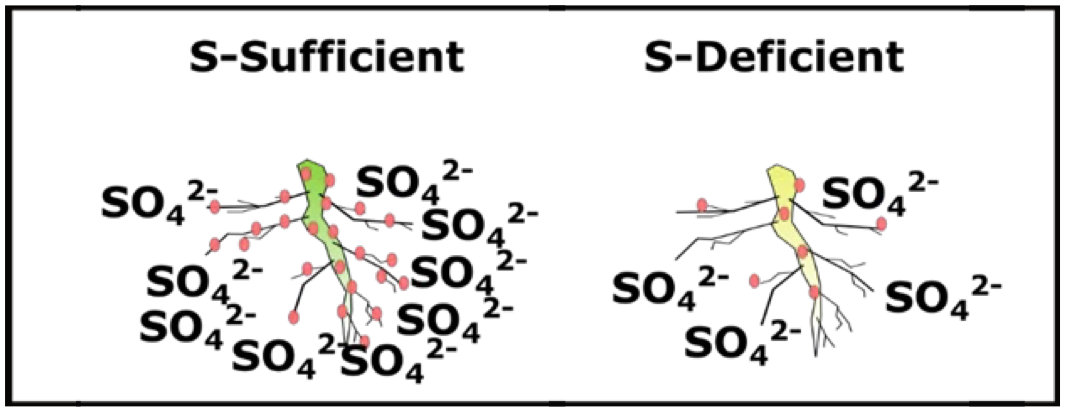
Low soil sulfate (SO4-2) reduces nodule formation on pea roots (Fig. 13), usually leading to less N fixation, which could lead to lower protein. In a greenhouse study, Zhao et al. (1999) compared N fixation rates in pea between 0 and 50 mg of S per pot. N fixation was consistently higher at 28, 38, 56, and 73 days after sowing when starter S was applied (Fig. 14). In addition, S is necessary for protein formation.
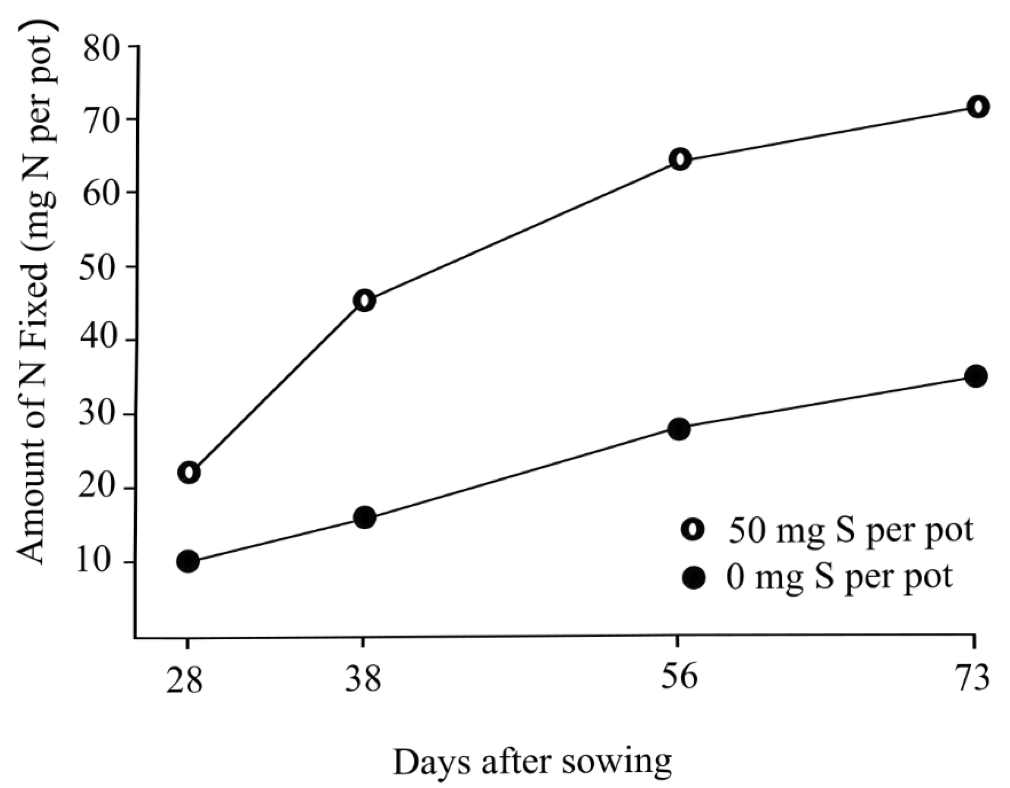
Starter S
Few field studies have addressed how S affects protein in pea. Bestwick et al., (2018) found slight correlation between pea protein and S management under moderate drought conditions in 51 pea fields across Montana. Across 52 sites in Alberta, increases in protein or yield were generally not observed between S rates of 0 vs. 18 lb/acre (McKenzie et al., 2001a). Spring soil S levels were not reported in this study, so it is possible that soil S was sufficient for protein. Soil tests are generally not a reliableindicator of S need in high gypsum soils or soils that have substantial sulfate below the usual two-foot soil test level. Prior crop performance, crop S removal rates, and tissue concentrations can be used in combination to evaluate S availability. On-farm experimentation may be the best method for Montana producers to test how starter S affects protein and yield in their fields.
K
Potassium indirectly affects pea development and N fixation similar to P. Montana soils are generally rich in K and on average exceed 250 ppm, though some soils have K levels as low as 70 ppm, especially in coarse, low pH soils (C. Jones, pers. comm.), and should be monitored in rotations with high removal of stems and leaves (forage, small grain straw). Pea yield or protein limitation by K deficiency has not been documented in Montana.
P, K, and S Management Summary
Soils deficient in P, S or K can reduce N fixation which could reduce protein and yield. A province-wide study in Alberta indicates that starter P can lead to modest increases in protein and yield, and increases are more likely to be realized when soil P tests are below 15 ppm. Alternatively, S did not increase protein or yield in Alberta but correlated modestly in Montana with grower-reported pea fields. Because most Montana soils are high in K, it is unlikely that additional K will boost protein and yield in most Montana soils. Bestwick et al. (2018) found no correlation between pea protein and P or K management across 149 fields in Montana.
Summary
Pea protein depends on N uptake and remobilization of N to the seed. Both drought and management interactions will likely affect these processes in Montana. Drought may reduce protein by decreasing N uptake, N fixation, and remobilization of N to the seed, but drought could increase protein by reducing seed number. Application of starter N will be most effective with low spring soil nitrate and under dry seedbed conditions. Inoculant is likewise beneficial with low soil nitrate levels and in fields where legumes have not been grown in the last five or so years. Granular inoculant could be more effective than seed-coat at increasing N fixation due to greater nodule distribution on tap and lateral roots. Starter P fertilizer may indirectly increase N fixation and will most likely be effective if soil P tests are low. Starter S may directly enhance N fixation, but soil S testing is unreliable in many soils, so on-farm experimentation may be the best way to determine if starter S benefits protein. It is unlikely that starter K affects protein on most Montana soils, though very limited research has been done on this topic.
Pea protein is a new study area for Montana. Canadian studies may have been conducted over more favorable growing conditions than what is typical in Montana, and their applicability to Montana production may be limited. For instance, recent statewide variety testing in Montana showed mean varietal yields (averaged across environment or site) ranged from 1995 - 2385 lb/acre (Mohammed et al., 2016). The studies from the Alberta and Saskatchewan reviewed in this document seldom reported yields below 2700 lb/acre (McKenzie et al., 2001a; Clayton et al., 2004b), suggesting drought did not limit pea production to the extent that would be expected in Montana. It is possible that drought and management interactions could affect pea protein differently in Montana than in Canada. The information provided here should therefore be considered as a starting point for adapting management to produce high pea protein.
References
Atta, S., S. Maltese, and R. Cousin. 2004. Protein content and dry weight of seeds from various pea genotypes. Agronomie 24: 257–266.
Bestwick, M., K. Olson-Rutz, C. Jones, and P. Miller. 2018. Environmental and Management Effects on Pea Protein in Montana: A Research Report from the Montana Research and Economic Development Initiative.http://landresources.montana.edu/soilfertility/documents/PDF/reports/Bestwick2018MREDIEnviron MgtPeaProt.pdf
Clayton, G.W., W.A. Rice, N.Z. Lupwayi, A.M. Johnston, G.P. Lafond, C.A. Grant, and F. Walley. 2004a. Inoculant formulation and fertilizer nitrogen effects on field pea: Nodulation, N2 fixation, and nitrogen partitioning. Can. J. Plant Sci. 84: 79–88.
Clayton, G.W., W.A. Rice, N.Z. Lupwayi, A.M. Johnston, G.P. Lafond, C.A. Grant, and F. Walley. 2004b. Inoculant formulation and fertilizer nitrogen effects on field pea: Crop yield and seed quality. Can. J. Plant Sci. 84: 89–96.
Guilioni, L., J. Wéry, and J. Lecoeur. 2003. High temperature and water deficit may reduce seed number in field pea purely by decreasing plant growth rate. Funct. Plant Biol. 30: 1151–1164.
Holl, F.B., and J.R. Vose. 1980. Carbohydrate and protein accumulation in the developing field pea seed. Can. J. Plant Sci. 60: 1109–1114.
Jakobsen, I. 1985. The role of phosphorus in nitrogen fixation by young pea plants (Pisum sativum). Physiol. Plant. 64(2): 190–196.
Jones, C. Professor/Soil Fertility Extension Specialist, Dept. Land Resources and Environmental Sciences, Montana State University, Bozeman.
Kutcher, H., G. Lafond, A. Johnson, P. Miller, K. Gill, W. May, T. Hogg, E. Johnson, B. Biederbeck, and B. Nybo. 2002. Rhizobium inoculant and seed-applied fungicide effects on field pea production. Can. J. Plant Sci. 82:645-651.
Larmure, A., and N.G. Munier-Jolain. 2004. A crop model component simulating N partitioning during seed filling in pea. F. Crop. Res. 85(2-3): 135–148.
Lhuillier-Soudele, A., N.G. Munier-Jolain, and B. Ney. 1999. Dependence of seed nitrogen concentration on plant nitrogen availability during the seed filling in pea. Eur. J. Agron. 11(2): 157–166.
Marino, D., P. Frendo, R. Ladrera, A. Zabalza, A. Puppo, C. Arrese-Igor, and E.M. Gonzalez. 2007. Nitrogen fixation control under drought stress. Localized or Systemic? Plant Phys. 143:1968-1974.
Markets and Market. 2015. Pea Protein Market by Type (Isolates, Concentrates, Textured), Application (Meat extenders and analogs, Snacks & bakery products, Nutritional supplements, Beverages), Textured Pea Protein by Form (Dry, Wet), & by Region - Global Trends & Forecast to 2020. http://www.marketsandmarkets.com/Market-Reports/pea-protein-market- 36916504.html?gclid=CP_s2vCu5swCFQWUfgodsSwJ8g.
McCauley, A.M., C.A. Jones, P.R. Miller, M.H. Burgess, and C.A. Zabinski. 2012. Nitrogen fixation by pea and lentil green manures in a semi-arid agroecoregion: Effect of planting and termination timing. Nutr. Cycl. Agroecosystems 92(3): 305–314.
McConnell, J.T., P.R. Miller, R.L. Lawrence, R. Engel, and G.A. Nielsen. 2002. Managing inoculation failure of field pea and chickpea based on spectral responses. Can. J. Plant Sci. 82: 273–282.
McKenzie, R.H., A.B. Middleton, E.D. Solberg, J. DeMulder, N. Flore, G.W. Clayton, and E. Bremer. 2001a. Response of pea to rhizobia inoculation and starter nitrogen in Alberta. Can. J. Plant Sci. 81: 637–643.
McKenzie, R.H., A.B. Middleton, E.D. Solberg, J. DeMulder, N. Flore, G.W. Clayton, and E. Bremer. 2001b. Response of pea to rate and placement of triple superphosphate fertilizer in Alberta. Can. J. Plant Sci. 81(4): 645–649.
McLean, L.A., I.F.W. Sosulski, and C.G. Youngs. 1974. Effects of nitrogen and moisture on yield and protein in field peas. Can. J. Plant Sci. 54: 301–305.
Miller, P. Professor, Dept. Land Resources and Environmental Sciences, Montana State University, Bozeman.
Mohammed, Y.A., C. Chen, K. McPhee, P. Miller, K. McVay, J. Eckhoff, P. Lamb, J. Miller, Q. Khan, B. Bohannon, M. Knox, and J. Holmes. 2016. Yield performance and stability of dry pea and lentil genotypes in semi-arid cereal dominated cropping systems. F. Crop. Res. 188: 31–40.
NASS. 2017. https://www.nass.usda.gov/Charts_and_Maps/Dry_Beans,_Dry_Peas,_and_Lentils/index.php, Washington, DC.
Padbury, G., S. Waltman, J. Caprio, G. Coen, S. Mcginn, D. Mortensen, G. Nielsen, R. Sinclair, and P. Albert. 2002. Agroecosystems and land resources of the northern great plains. Agron. J. 94: 251– 261.
Rennie, R.J. and R.K. Hynes. 1993. Scientific and legislative quality-control of legume inoculants for lentil and field pea. J. Prod. Ag. 6: 569-574.
Schiltz, S., N. Munier-Jolain, C. Jeudy, J. Burstin, and C. Salon. 2005. Dynamics of exogenous nitrogen partitioning and nitrogen remobilization from vegetative organs in pea revealed by 15N in vivo labeling throughout seed filling. Plant Physiol. 137(4): 1463–1473.
Sosulski, F.W., L.A. Mclean, and H.M. Austenson. 1974. Management for yield and protein of field peas in Saskatchewan. Can. J. Plant Sci. 54: 247–251.
Tao, A., R.K. Afshar, J. Huang, Y.A. Mohammed, M. Espe, and C. Chen. 2017. Variation in yield, starch, and protein of dry pea grown across Montana. Agron. J. 109: 1491-1501.
Zhao, F., A. Wood, and S. McGrath. 1999. Effects of sulphur nutrition on growth and nitrogen fixation of pea (Pisum sativum L.). Plant Soil 212: 209–219.
