Soil Fertility Basics
Plants require 17 nutrients to grow and reproduce, and insufficient amounts of these
nutrients can limit
plant growth. Three non-mineral nutrients acquired from air and water (carbon, hydrogen,
and oxygen) generally
don’t limit plant growth. The six macronutrients (nitrogen, phosphorus, potassium,
calcium, magnesium, and sulfur) are needed in larger quantities, with the “primary”
macronutrients nitrogen (N), phosphorus (P) and potassium (K) most commonly limiting
plant growth. Of the eight micronutrients, which are needed in smaller
amounts, boron (B), chlorine (Cl), copper (Cu), iron (Fe), manganese (Mn), sulfur
(S) and zinc (Zn) deficiencies
have been observed in Montana, while molybdenum (Mo) and nickel (Ni) have not.
Soils have large quantities of most nutrients, yet the majority of these nutrients are not in soil water and cannot be readily taken up by plants. Instead, they are in minerals, organic matter, or are “exchangeable nutrients” that are held to soil particles by weak electric charges.
Cation Exchange Capacity
Soil has an overall negative electric charge because clay and organic matter (OM)
in soil have many more negative
charges on their surfaces than positive charges. Positively charged nutrient cations
are attracted and held to these
negatively charged surfaces like a magnet, removing nutrients from soil water. These
attractions are weak, so as nutrients are taken up by a plant, more nutrients held
to soil can release and become available (Figure 1). Cation exchange capacity (CEC)
is a measure of how much negative charge is in a soil. A higher CEC soil can hold
more nutrients, minimizing nutrient losses due to water leaching them out, and is
a good indicator of soil fertility.
Clays, the smallest soil particles, have a high CEC because they have more exposed
surface area to hold nutrients
than silts and sands and because of chemical changes that happen as clay forms. Like
clay, organic matter has a high surface area and a high CEC. In addition, OM retains
water and releases nutrients as it decomposes, functioning as a slow-release fertilizer.
Soils can also hold anions, which are negatively charged particles, but anion
exchange capacity (AEC) is substantially smaller than CEC and less important for soil
fertility.
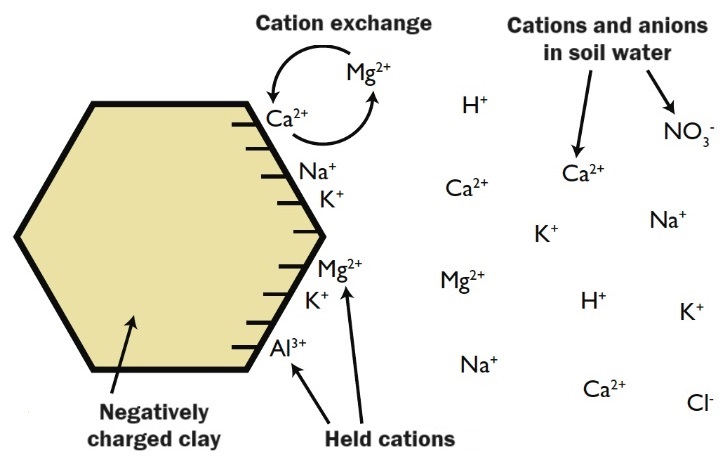
Figure 1. A simplified representation of cation exchange capacity on a clay particle (adapted from Brady and Weil, 2002; see Soil & Water Module 1 for reference).
Soil Texture and Nutrient Mobility
There are 12 major soil textures determined by the percentage of sand, silt, and clay in a soil (Figure 2). Sands are larger soil particles (0.5 to 2.0 mm), clays are the smallest (invisible to the eye, <0.002 mm), and silts are in between. Texture strongly influences plant nutrition by affecting its CEC and the movement of water and nutrients. Sandy soils have a low ability to hold nutrients; sand has less surface area to hold nutrients, isn’t negatively charged, and compacts loosely in soil, creating large, empty pore spaces that allow water to drain out and remove nutrients. On the other hand, soils high in clays have larger surface areas, high CECs, and are tightly packed with smaller pore spaces that hold water and nutrients in place like a sponge.
Nutrients vary greatly in their relative mobility within a soil. There are two ways
nutrients can move to plant roots:
mass flow and diffusion. Mass flow is when nutrients are transported by the movement
of water. Diffusion is a
much slower process, where nutrients move from areas of high nutrient concentration
to low concentration
(similar to food coloring spreading in still water until it is evenly mixed). Nitrogen
and sulfur are very mobile and move through mass flow, which makes them prone to leaching
out of sandy soils, but they can also reach plant roots more easily and can be applied
mid-season. Phosphorus is immobile and must slowly diffuse to roots for plants to
access it, so phosphorus fertilizer needs to be placed near seeds during planting
to be available in the current growing season.
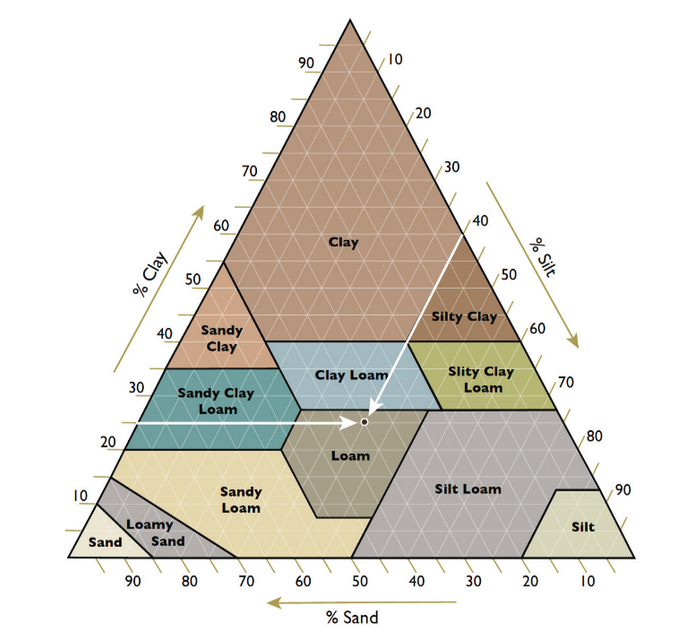
Figure 2. Texture triangle showing soil textural classes according to the percentages of sand, silt and clay. A soil with 35% sand, 25% clay and 40% silt is loam.
Soil pH
The pH of a soil is a measure of the soil’s acidity based on its hydrogen (H+) concentration.
Soils can be alkaline/
basic (pH > 7), neutral (pH near 7), or acidic (pH < 7). Soil pH affects the availability
of all nutrients.
Base cation nutrients (Na+, K+, Ca2+, Mg2+) hold more weakly to soil at low (acid)
pH, so they can leach out of
acid surface soils and become less available. The high concentration of H+ displaces
nutrients and neutralizes
the negative charges on clays and organic matter. The optimum pH appears to be near
7, but every plant has
different nutrient needs and optimum pH levels.
Metal toxicity can also become an issue for plants at lower pH levels. Metals like
Cu, Fe, Mn, and Zn are less available at high pH because metals are held very tightly
to the soil or form solid minerals. Fertilizing with ammonia-based fertilizers is
one way that pH decreases and soils become acidic over time. An unpublished study
by Agvise Laboratories, Inc. found the number of Montana soil samples with pH less
than 6 doubled between 2002
and 2022 (Northwood, North Dakota).
Roots and Mycorrhizae
Both plant roots and mycorrhizae, which are beneficial fungi that colonize plant root systems, exude acids that release nutrients from clays into soil. Mycorrhizae grow their own roots (“hyphae”) which increase the surface area of soil accessed and help with nutrient and water uptake, in exchange for energy supplied by the host plant.
Some crops, such as corn, are highly dependent on this association, while others (e.g., canola) cannot support mycorrhizal fungi.
For more information:
Soil and Water Management Modules
• Module 1: Basic Soil Properties
Nutrient Management Modules
• Module 2: Plant Nutrition and Soil Fertility
• Module 8: Soil pH and Organic Matter

