Soil Acidification: Background
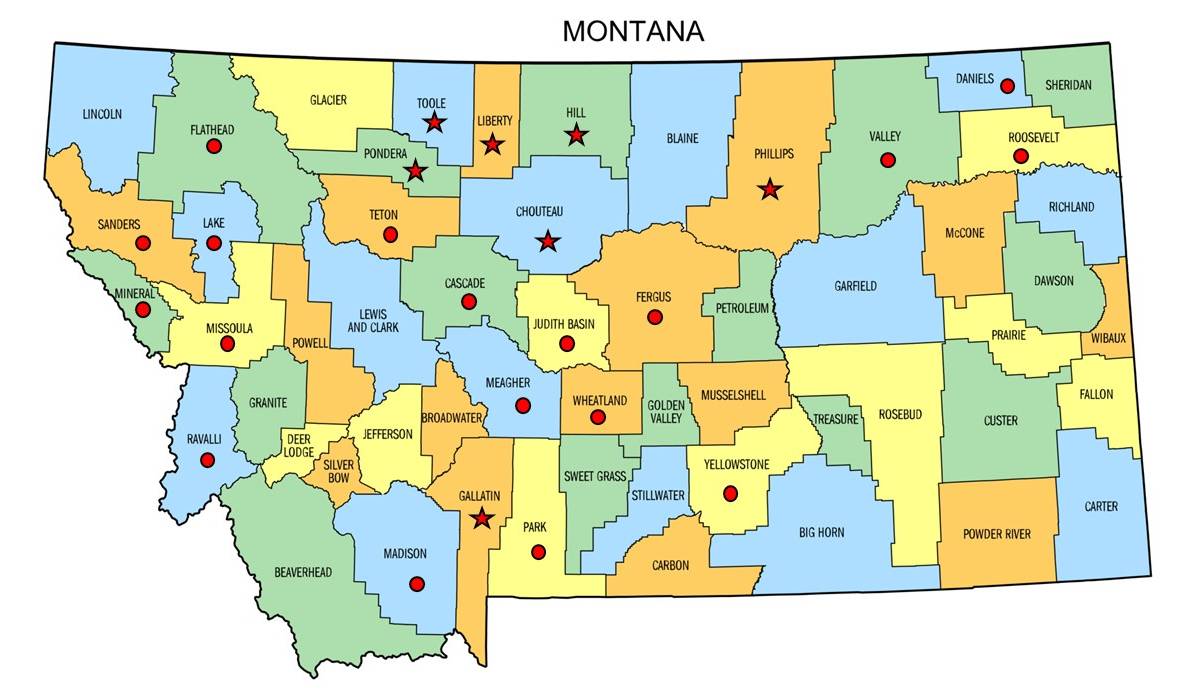
Soil pH test results reveal that Montana's agricultural fields are becoming more acidic; and the effects are not confined to any single county but occur in many agricultural regions of the state, including those which are traditionally known to have high pH soils (Figure 1).
Figure 1. Montana counties in which fields with soil pH < 5.5 have been found as of May 2024. Symbol is not the location of that field.
The agronomic concerns of low soil pH are multiple:
- aluminum and manganese toxicity
- nutrient deficiency
- poor N fixation in legumes
- increased fungal disease
- changes in herbicide effectiveness and carry over
These all contribute to decreases yields or at worst, crop failure (Figure 2).

Figure 2. A field in Chouteau county, MT, seeded to durum wheat and experiencing aluminum toxicity symptoms (soil pH = 4.5). May 18, 2016. Image courtesy R. Engel.
There are soils which naturally have low soil pH. These include soils with low buffering capacity due to low soil organic matter, coarse texture, or granitic rather than calcareous parent material. Soils historically supporting forest rather than grassland ecosystems tend to have lower pH. The agronomic development of soil acidity problems in Montana can be traced to:
- increase in ammonium-based N fertilizer use, especially if above crop needs (Figure 3)
- leaching loss of nitrate (NO3-)
- crop residue removal
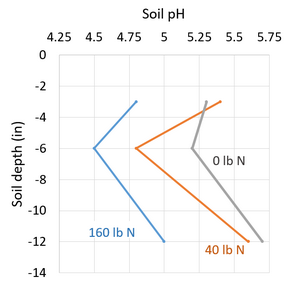
Figure 3. Urea subsurface band for 9 years at 6" depth at greater than the suggested 40 lb N/acre reduced soil pH down to 12" depth (Bouman et al., 1995, SK).
More information is available in the two Soil Scoops Soil Acidification: Problems, Causes & Testing and Soil Acidification: Management, as well as in other items listed on our Resources page.
References cited:
Bouman, O.T., D. Curtin, C.A. Campbell, V.O. Biederbeck, and H. Ukrainetz. 1995. Soil acidification from long-term use of anhydrous ammonia and urea. Soil Sci. Soc. Am. J. 59:1488-1494. doi:10.2136/sssaj1995.03615995005900050039x